Faulty of Engineering School of ChemicalPetroleum Engineering Chemical Engineering department Laboratory of thermodynamics EXPERIMENT NUMBER TWO Boyles law expansion Instructor. To determine the total initial volume of your apparatus V i take it to a deep sink close.

Conclusion For Gas Laws Docx Conclusion In This Lab We Tested How Pressure Volume And Temperature Of Gases Are Related We Found Out Our Results By Course Hero
For a fixed amount of gas kept at a constant temperature PV k.

. This is important because the more pressure an object has the less volume it has. 29oct2013 Report Submitted on. He made these observations by using Mercury in a J-tube and made measurements of the volume of the trapped gas at pressures both higher and lower then.
P is the pressure V is the volume and K is the constant. This experiment was meant to explore the relationship between pressure and gasses described by Boyles Law. To determine the relationship between pressure and volume of an ideal gas.
Shwan sarwan sadiq Experiment Contacted on. Boyle Report Lab Khogr kamal Ibarhim Second Class Production and metallurgy engineering 2018-2017 2. Note that if T is held constant throughout the experiment then the ideal gas law reduces to Boyles law.
Historically this relationship was first established by Robert Boyle in 1662 and has since been known as Boyles law. Ideal gas is the gas which composed by many moving molecule and has an elastic collision between the particles. Students will investigate Boyles law using syringes gas pressure sensors and labquest digital data collection units.
APPARATUS Pressure sensor syringe and computer. HttpsyoutubeJ9IoAp51_7w in which the relationship between gas pressure and volume. The purpose of this experiment was to understand Boyles LawIn the experiment the pressure in the system under constant temperature and mass was used to confirm if the laws are trueBoyles law relates pressure and volume while all other factors are consistent and states.
PROCEDURE Record the posted values of the barometric pressure and room temperature at the time of the experiment. BOYLES LAW OBJECTIVE To determine the relationship between pressure and volume of an ideal gas. Boyles law is a pressure versus volume relationship.
Experiment No- 1 Experiment Name -Boyles law lussas law Objective- 1- Demonstrating the laws of state changes in gasses experimentally 2- Isothermal change of state Boyles law. Therefore P1V1 k initial pressure initial volume P2V2 k final pressure final volume P1V1 P2V2. This is also known as Boyles Law which can be dated back to 1662.
Ankon Rahman Introduction Boyles law is an established law of chemistry given by Robert Boyle 1627-1691. INSPECT AND REPORT Have the carrier note the specific damages on the freight bill BEFORE accepting delivery. This law of chemistry is one of the gas laws of chemistry.
This experiment and then analyzed with LoggerPro. Boyles Law is identified through the equation PVk. Analysis of data obtained in the Boyles law experiment video here.
Robert Boyle discovered the inverse relationship between pressure and volume in 1662. Independent variable Mass m kg Dependent variable Volume V m 3 Control. Boyles law expansion 1.
An Introduction To Boyles Law. This expression can be obtained from the pressure-volume relationship suggested by Boyles law. In addition to data this document contains the revisions to the usual procedure to help you adjust for online.
As the pressure increases the volume decreases and vice versa. Volume of a Gas at Constant Temperature shows you a simple method for re-creating Boyles famous experiment. Boyles Law Lab Report.
Open the valve and pull the plunger back to set the initial volume of the air in. 3- Isochoric change of state gay lussas law History of boyles law- Boyles law. This equation can be used to predict the increase in the pressure.
To compare the experimental results with theoretical results. For a fixed amount of gas kept at constant temp the product of the. Connect the open end of the syringe to the pressure sensor.
Boyles Law Lab Report. Glass tube syringe 100 mL H 2O V w 50 mL initial H 2. Boyles Law Lab Report 1.
From the data and graph the mathematical relationship between the pressure and volume of the confined gas will be identified. References THERMODYNAMICS LAB REPORT TITL E. All Chemistry Classes Boyles Law Lab Name _____ Purpose.
Graphs of Boyles law. Boyles Law Science Fair Experiment. Students will then complete a formal lab report.
Mr rebwarMr umer Author Name. The Ideal Gas Law is the equation of state of an ideal gas. Lab Report Boyles Law Task 1 PART A.
Your lab report will consist of your data sheet. Put the plunger back and make sure it. If possible take a picture of the damages for your claim with your.
This is just one example of how this required practical might be tackled. This relationship is now known as Boyles Law. Boyles Law Science Fair Experiment.
Pressure Sensor Figure 1 PRELIMINARY QUESTIONS 1. Boyles experiment A very detailed description of the historical experiment that led to Boyles law can be found in the Part II chapter V of the Boyles book published in 1662 6 and also in the liter-ature 12. The overall aim of this experiment is to investigate the effect of Boyles Law This is the effect of pressure on volume at a constant temperature.
Discussion For the Boyles Law experiment conducted the purpose is to study the variation in volume with pressure for a sample of air in a closed container at constant temperature by plotting. To keep the experiment as accurate as possible add a few drops of silicone lubricant into the syringe. EXPERIMENTAL PROOF OF BOYLES LAW OCTOBER 3 2012 LAB PERFORMED BY.
The gas tend to act like an ideal gas in high temperature and. Boyles Law states that pressure and volume are inversely proportional to each other. Using Boyles law explore the relationship between pressure and the volume of a gas.

Lab 11 Pt1 Xlsx Virtual General Chemistry Laboratory Name Gas Laws Date 07 15 2017 Experiment 1 Boyle S Course Hero

A Simple Demonstration For Ideal Gas Laws

Experiment 9 Gas Laws Name Section Date Data Lab Chegg Com
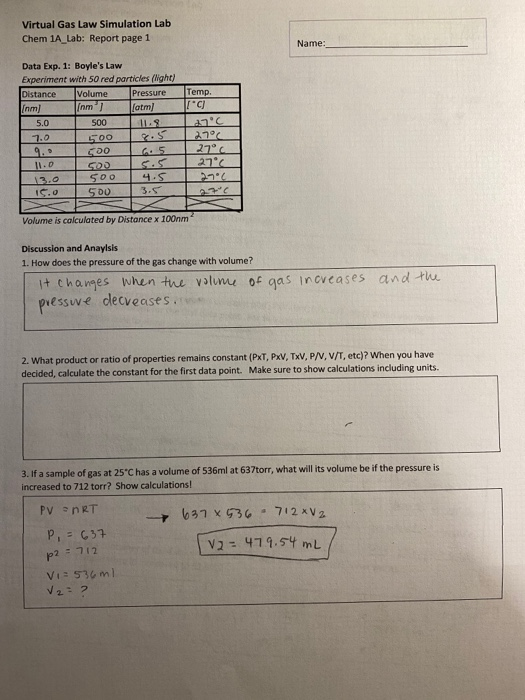
Solved Virtual Gas Law Simulation Lab Chem 1a Lab Report Chegg Com

Key 8 1 Gas Law Lab Gas Lab Key For Chapter 8 Information 8 Discovery Lab Gas Laws Purpose To Studocu

Boyles Law 2 Pdf Pressure Gases

10 Boyles Law Short Answer Docx Boyle S Law Question 1 Experiment 1 Record The Pairs Of Data For Pressure And Propane Volume In The Table Course Hero

Lab 11 Pt1 Xlsx Virtual General Chemistry Laboratory Name Gas Laws Date 07 15 2017 Experiment 1 Boyle S Course Hero

Lab 11 Pt1 Xlsx Virtual General Chemistry Laboratory Name Gas Laws Date 07 15 2017 Experiment 1 Boyle S Course Hero

Solved Thermodynamics Lab 1 Please Download Ideal Gas In 3d Chegg Com

Perfect Gas Law Lab Report Pdf Gases Pressure
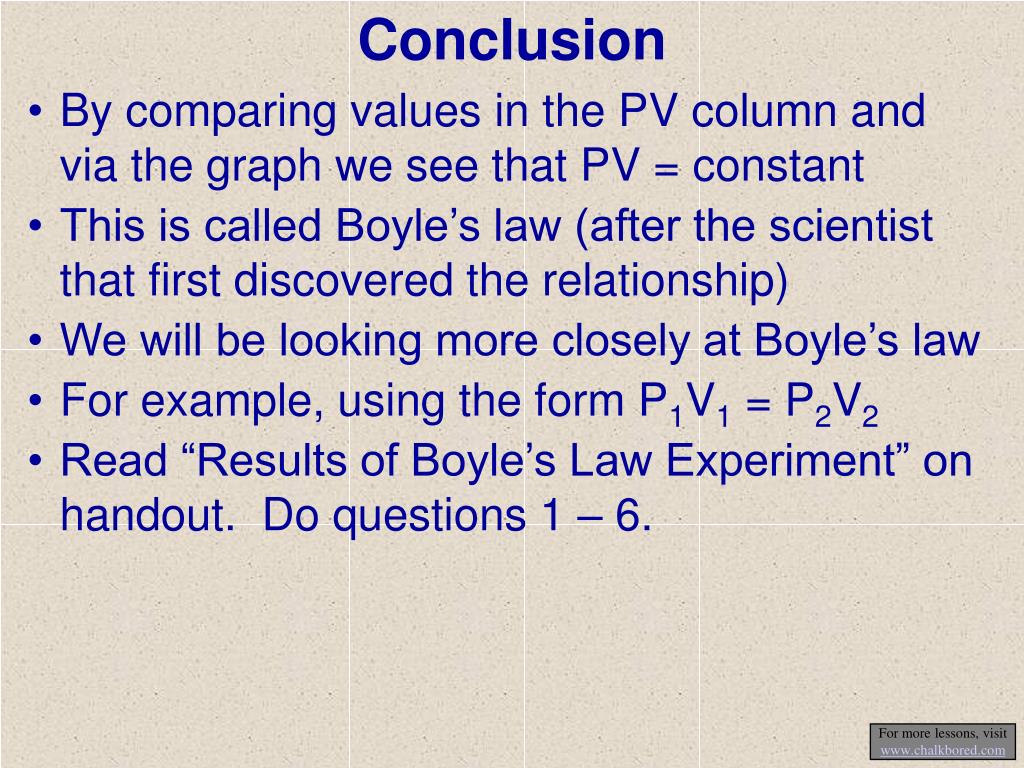
Ppt Boyle S Law Lab Powerpoint Presentation Free Download Id 352982

Lab 11 Pt1 Xlsx Virtual General Chemistry Laboratory Name Gas Laws Date 07 15 2017 Experiment 1 Boyle S Course Hero

Experiment 9 Gas Laws Name Section Date Data Lab Chegg Com

Perfect Gas Law Lab Report Pdf Gases Pressure

Boyles Law 2 Pdf Pressure Gases



